Adcon Gel | anti-adhesion gel
Adcon® Gel is a biocompatible, resorbable gel that provides a physical barrier to intertissue adhesions and inhibits fibroblast migration.
This results in improved patient rehabilitation, reduced recurrent pain, and decreased number of re-operations as a result of such adhesions.

Adcon Gel anti-adhesion gel
- Adhesion control: prevents and controls adhesion formation
- Resorbable: gradual resorption over a period of four to six weeks
- Extensive clinical history: used in over 300,000 cases worldwide and in more than 10 clinical studies since 1996
- Ease of use: special applicator for easy, secure application of the gel
- Indicated for spinal decompression surgeries (laminectomy, laminotomy) and spinal fusion
Benefits of Adcon® Gel
Adcon® Gel is a biocompatible resorbable gel, composed of polyglycan ester combined with absorbable gelatine in a phosphate-buffered saline.
- Prevents adhesion formation: physical barrier to inter-tissue adhesions, adheres to the tissues it is applied onto, and inhibits fibroblast migration on and around neural tendinous structures
- Resorbable: biocompatible gel that gradually resorbed over a period of 4-6 weeks
- Adcon® Gel reduces and simplifies re-operations, resulting in lower incidence of Failed Back Surgery Syndrome (FBSS) and eliminates the need to perform differential diagnostics (X-rays, MRIs)
- Extensive clinical history: used in over 300 thousand cases worldwide and in more than 10 clinical studies since its introduction in 1996
- Ease of use: special applicator for easy, yet secure application of the gel to difficult to reach or irregular structures
Intra-operative vertebral area showing nerve roots, dura and ligaments free of scar tissue.
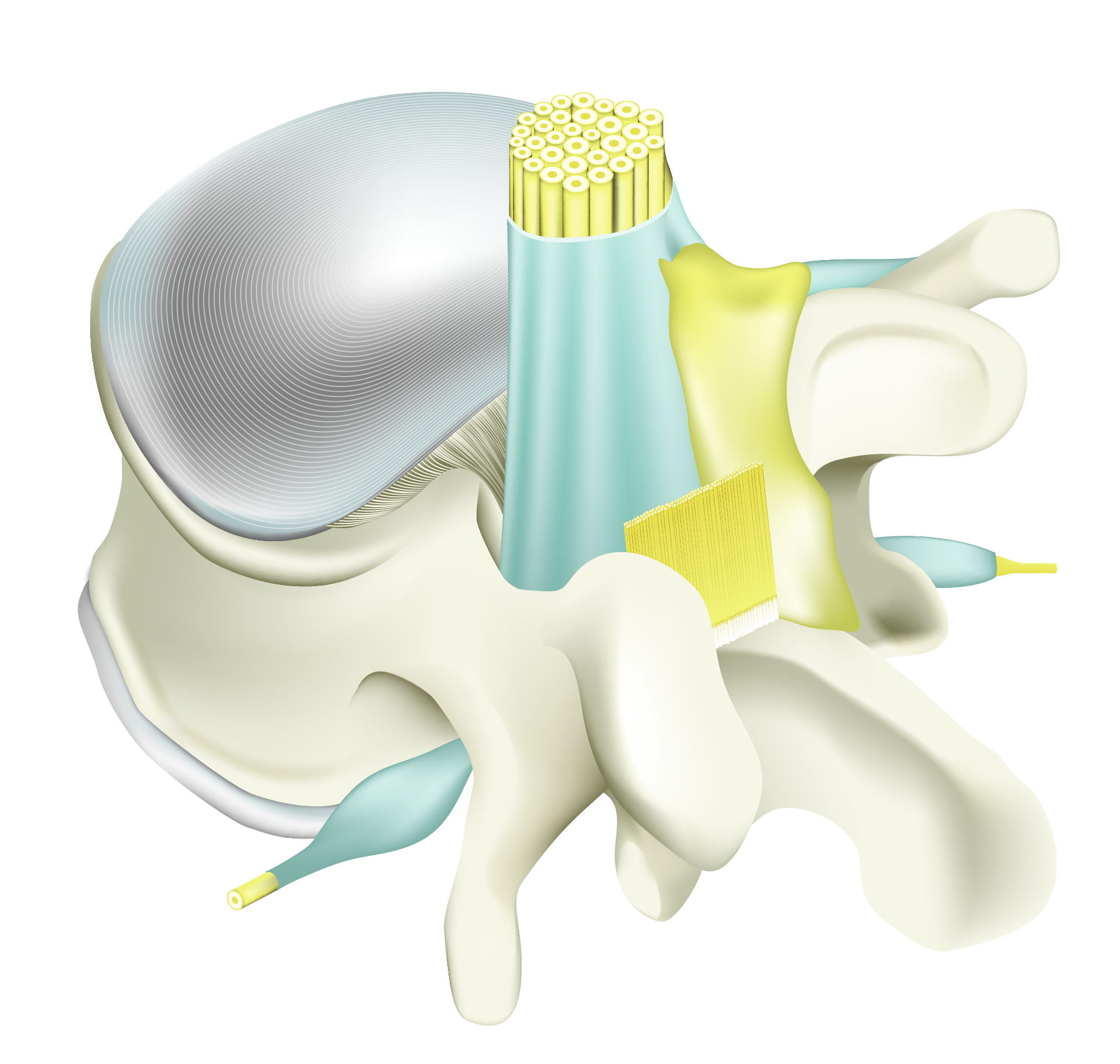
Adcon Gel applied
Adcon® Gel surrounds the nerve root, dura and the ligaments minimising adhesion.
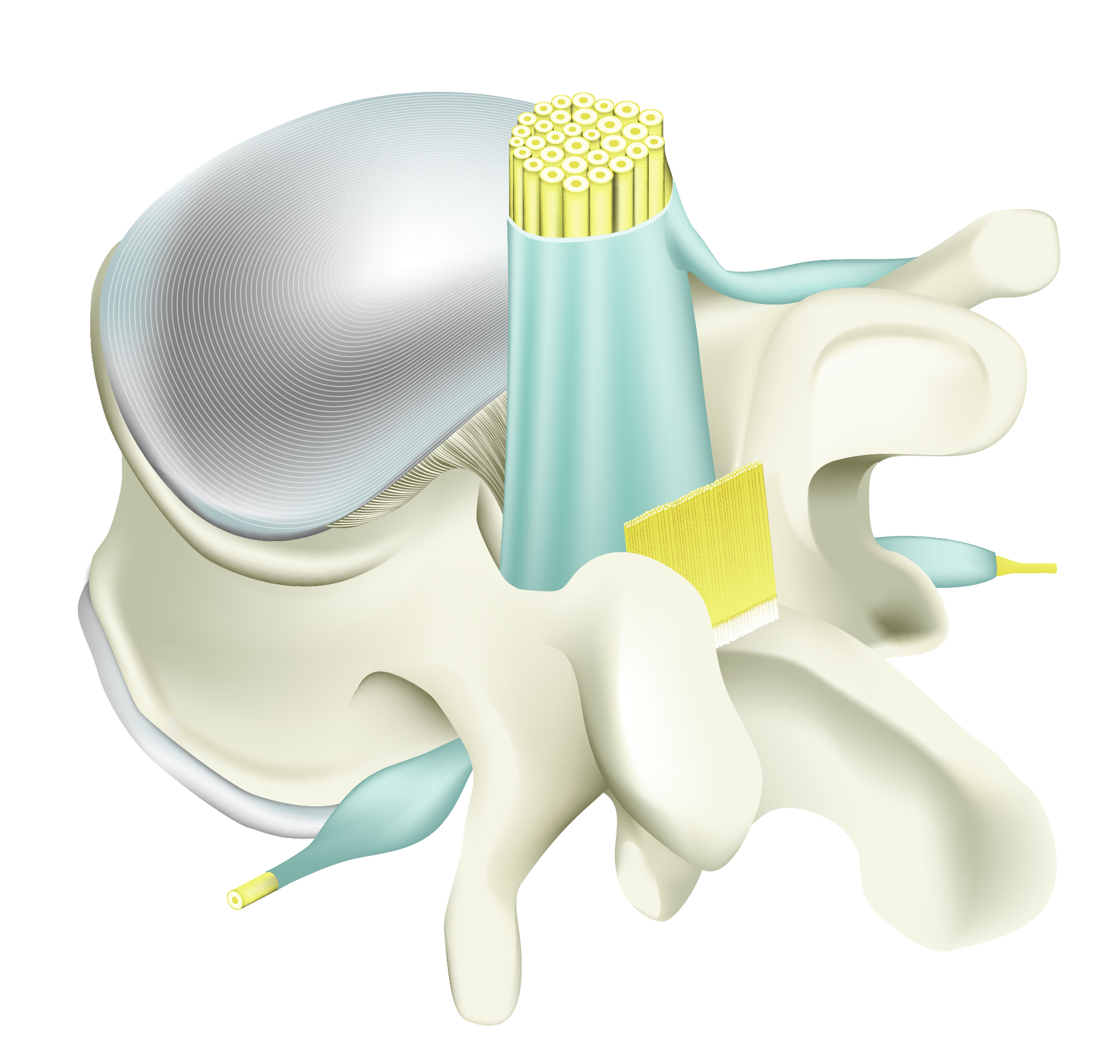
Untreated post-operative vertebral area showing adhesions around operated area: dura, nerve root, ligament and soft tissues.
All products are subject to registration and availability, and may vary by country. Consult your local Leader Biomedical contact or email us at info@leaderbiomedical.com

 Global
Global  India
India APAC
APAC Nederland
Nederland