From developing a robust process to scaling-up by starting production: utilise our eCOO® Developer Packages to establish proof of concept, confirm viability, and conclude with a product launch to your preferred market.
Once the process is optimized for your material, we can perform in-depth verification studies to confirm robustness for up-scaling into a production setting. Here we finalize a fitting production protocol to develop your material according to your product specifications. Necessary data is generated to support your proof of concept. Relevant, extensive microbiology testing and evaluation methods will be defined. Following this, we can support you by creating an executing validations using parameters justifying the process for compliancy with the standards required, such as ISO13485, GMP and ISO11737.
Developer Standard | Developing proof of concept
For research institutes and start-ups: the Developer Standard Package includes defining optimised protocol and proof of concept data with relevant sample sizes and microbiology/sterility testing, compliant to standards.

Developer Plus | Production and promotion of sellable product
Ideal for start-ups: the Developer Plus Package offers extensive support and service for the implementation of product manufacturing including technical file studies and compliant to standards with additional regulatory and marketing support provided.
CASE SPOTLIGHT:
REDUCING RISKS ON COLLAGEN PRODUCTS WITH eCOO® CLEAN
Medtech start-up applied eCOO® Clean as processing technology on animal derived serous membrane

The objective of the start-up was to expand their portfolio and enter the US market by developing an FDA approved collagen membrane to support dental bone augmentation.
A concept of a safe and effective product was envisioned that would result in excellent regeneration performance and ensure safety of the patient. Furthermore, the regulatory pathway was to be kept as short as possible and supply available for the magnitude of the market.
To achieve this native porcine pericardium tissue was processed with eCOO Clean®, which resulted in a naturally cross linked, safe and effective collagen membrane. As the technology was only slightly adapted for this tissue type, optimizations were quickly established into scalable production protocols and data generation could be initiated quickly with limited risk of failure.
This trajectory resulted in relatively fast production of compliant product and completion of the relevant technical file, as well as a high-quality product. 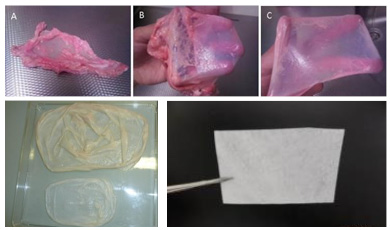
“The company succeeded in production of the envisioned product and launch it in the region faster than building in-house production and going through the regulatory pathway”
You may also be interested in reading these Clinical Cases
- Dillow A.K. et al. (1999) Bacterial inactivation by using near- and supercritical carbon dioxide, Proc. Natl. Acad. Sci. USA, Vol. 96, pp. 10344–10348
- Fages J., et al. (1998) Viral Inactivation of Human Bone Tissue Using Supercritical Fluid Extraction, ASAI Journal
- Russel N., et al. (2013) The effect of sterilization on the dynamic mechanical properties of paired rabbit cortical bone, Journal of Biomechanics 46, 1670–1675
- Shieh E. et al. (2009) Sterilization of Bacillus pumilus spores using supercritical fluid carbon dioxide containing various modifier solutions, Journal of Microbiological Methods 76, 247–252
- Vang P., Day D. (2006) Advantages and Disadvantages between Allograft versus Autograft in Anterior Cruciate Ligament Replacement, Dept. of Physicians, College of Health Professions

 Global
Global  Nederland
Nederland APAC
APAC India
India